
Recently, Stardust Technology completed the registration of the first medical device master file for spherical tantalum powder for additive manufacturing orthopedic implants and obtained the registration certificate issued by the National Medical Products Administration. This important milestone marks our deep strength and continuous innovation in the field of medical devices.
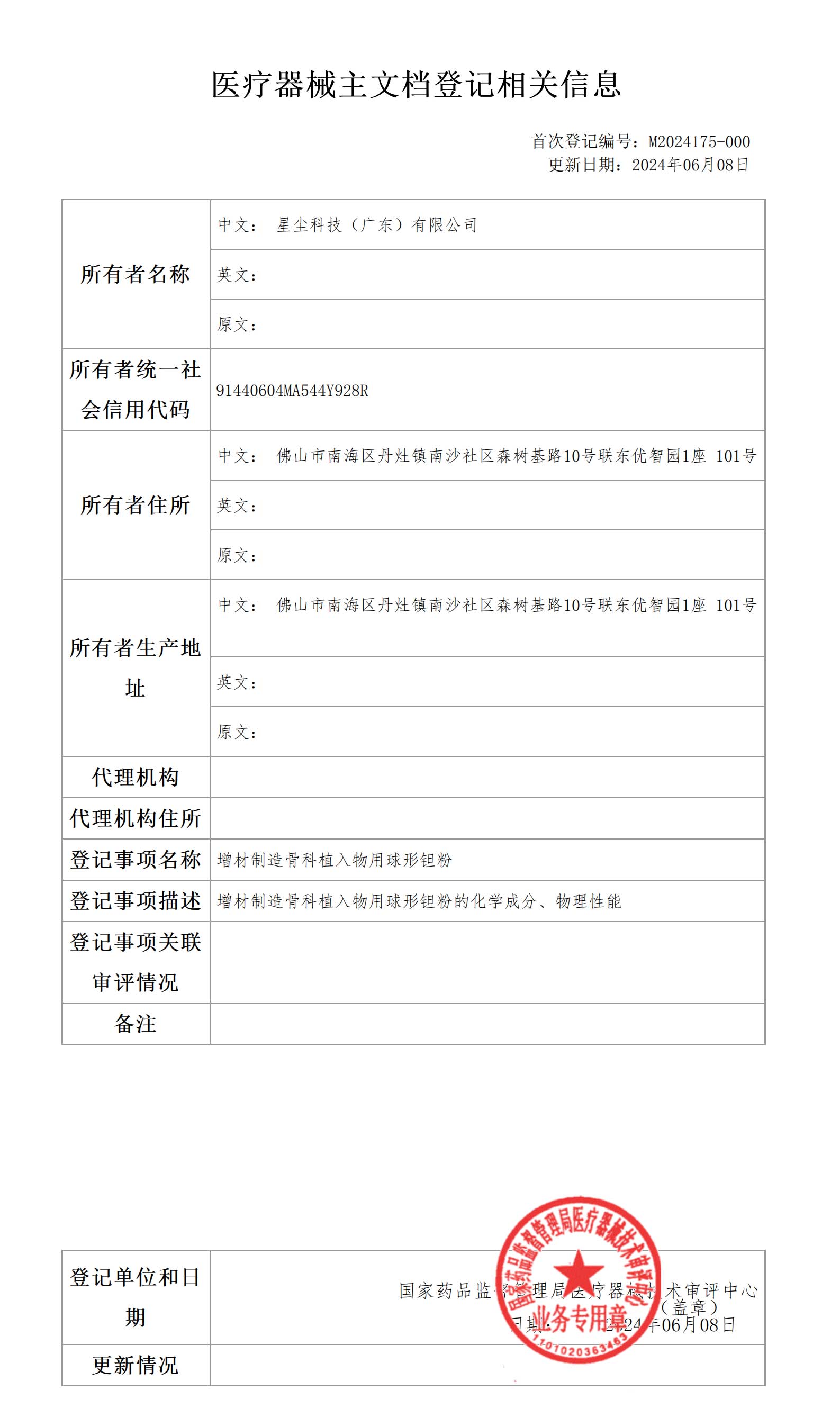
The spherical tantalum powder produced by Stardust Technology using radio frequency plasma spheroidization technology is widely used in 3D printing of clinical medical implants such as spine, joints, and trauma due to its excellent biocompatibility and bone ingrowth characteristics. Stardust Technology was the first in China to achieve an industrial breakthrough in medical-grade spherical tantalum powder, and jointly promoted the clinical medical application of tantalum metal with domestic first-class orthopedic hospitals and medical device companies. It has participated in the formulation of 2 relevant national standards, 1 industry standard, and 3 group standards, and assisted in the completion of more than 500 cases of clinical application of tantalum metal.

Stardust Technology has always been committed to the combination of technological innovation and industrial application. In the future, we will bring more technological breakthroughs, continue to inject new vitality into the medical device industry, and lead a new era of medical devices!


